Research thrust II
SINGLE CELL MANIPULATION & ANALYSIS
Manipulating cells (e.g., reprogramming and differentiation of stem cells) through delivery of biomolecules (RNA, proteins, plasmids) is transforming drug discovery, disease study, and synthetic biology. We design and build nano platforms for single cell access and analysis, cell-cell interactions, and mechano-transduction in cellular response.
AREAS OF FOCUS
MICROFLUIDIC PLATFORMS FOR GENE EDITING
MICRO & NANO TECHNOLOGIES FOR CELL ANALYSIS
BIOPHYSICS OF INTRACELLULAR DELIVERY
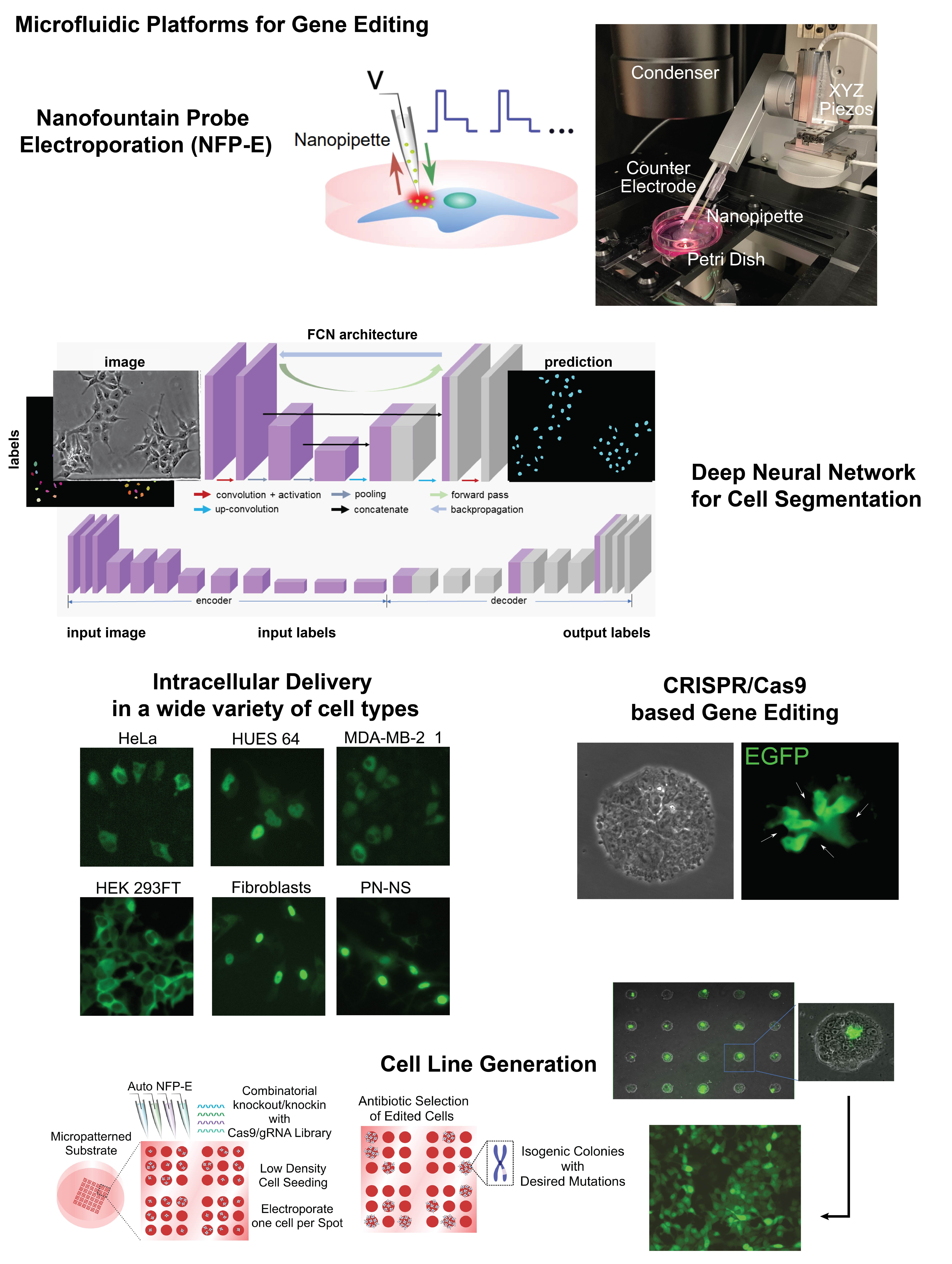
MICROFLUIDIC PLATFORMS FOR GENE EDITING
Genome engineering of cells using CRISPR/Cas systems has opened new avenues for pharmacological screening and investigating the molecular mechanisms of disease. A key step in such studies, is the intracellular delivery of the gene editing machinery. However, most of the current delivery methods such as bulk electroporation or chemical carriers are harsh to sensitive cell types. This often leads to poor viability and low overall efficacy, requiring the use of large starting samples.
Our group focusses on developing novel microfluidic platforms for minimally invasive and non-toxic delivery of molecular cargo into a wide variety of cell types. Two such platforms are described below:
- The Nanofountain Probe Electroporation (NFP-E) System is a nanopipette-based single-cell electroporation method that provides superior cell viability and dosage control compared to traditional methods. The automated system utilizes a convolutional neural network (CNN) to identify cell locations and a cell-nanopipette contact algorithm to position the nanopipette over each cell for the application of electroporation pulses (see Video). We demonstrated, using a wide range of cell types and molecular cargos, that the NFP-E system minimally perturbs cells during intracellular delivery. Current work is focused on combining the automated NFP-E with micro-confinements for cell isolation, in-situ CRISPR/Cas9 gene editing, and combinatorial manipulation of single cells for the generation of isogenic cell lines.
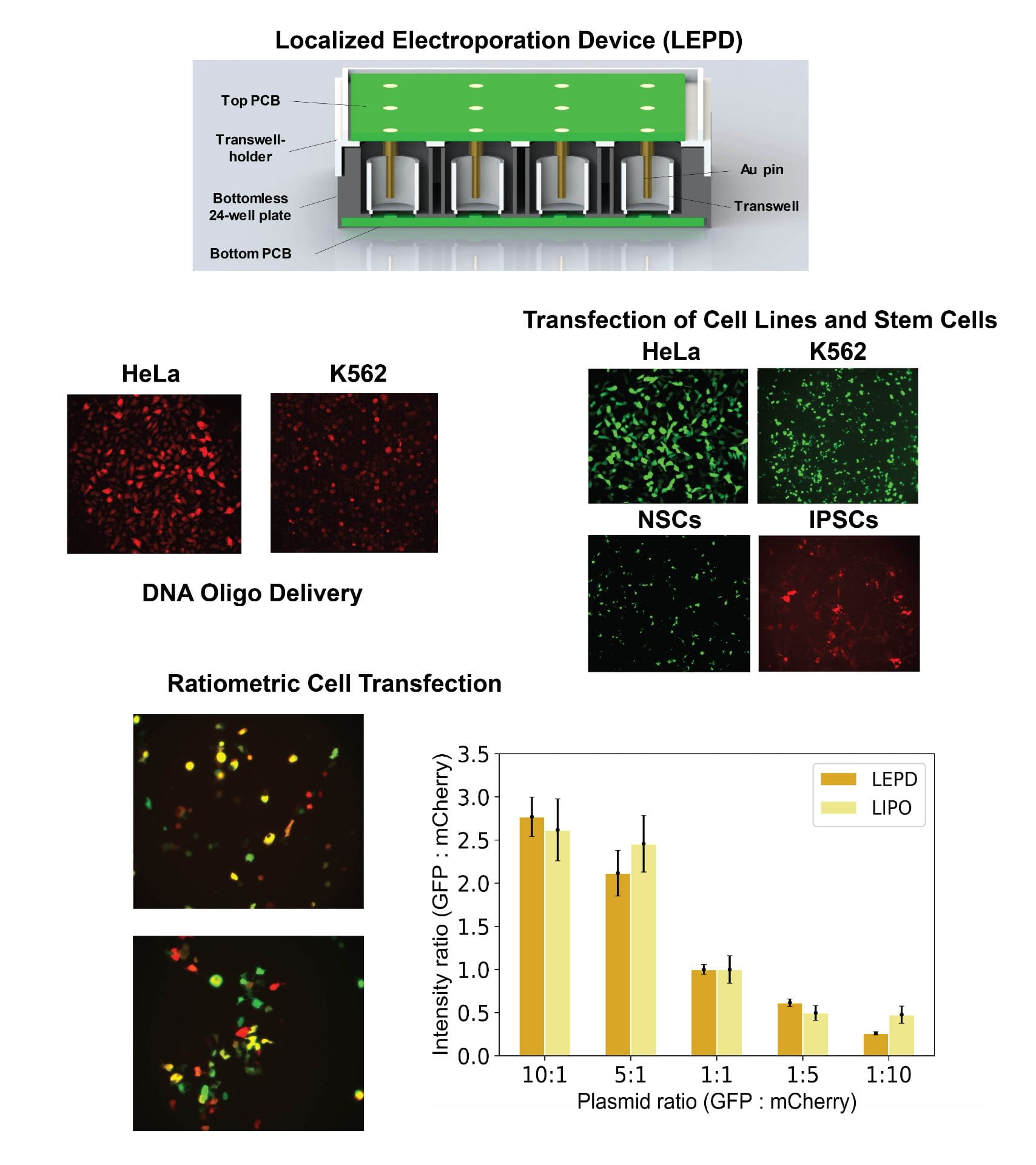
- The Localized Electroporation Device (LEPD) is a microfluidics platform that enables long term cell culture, automated imaging assays and high-throughput intracellular delivery via localized electroporation. In the LEPD, cells are plated on nanoporous polymer membranes. When an electric field is applied across the LEPD, the nanopores in the substrate confine the electric field to a small fraction of the cell membrane and minimize perturbations to the cell. The multi-well plate inspired format of the LEPD allows for multiplexing different experimental conditions. We have used this platform to transfect and culture sensitive cells (primary/stem cells) while preserving a high degree of cell-viability. Current studies using the LEPD include editing Hematopoietic Stem and Progenitor Cells (HSPCs) with Cas9 RNP for sickle cell mutation (beta-globin gene) correction and comparison of LEPD performance with existing cell transfection technologies.
*Video: Automated NFP-E operation
MICRO AND NANO TECHNOLOGIES FOR CELL ANALYSIS
Advances in molecular biology, automated instrumentation, and data analysis techniques allows investigation of molecular states of individual cells with unprecedented detail. These new technologies can be used to connect genotype and regulatory factors to phenotype, in both natural and diseased states. However, these methods rely on cell lysis and can provide only a snapshot of the cellular activity. This makes it challenging to monitor gene expression over time, which is key to understanding the fundamental mechanisms governing dynamic cellular processes such as differentiation, maturation, and ageing. Moreover, methods of introducing cellular perturbations often have deleterious effects on sensitive cells, that can obfuscate downstream measurement and analysis.
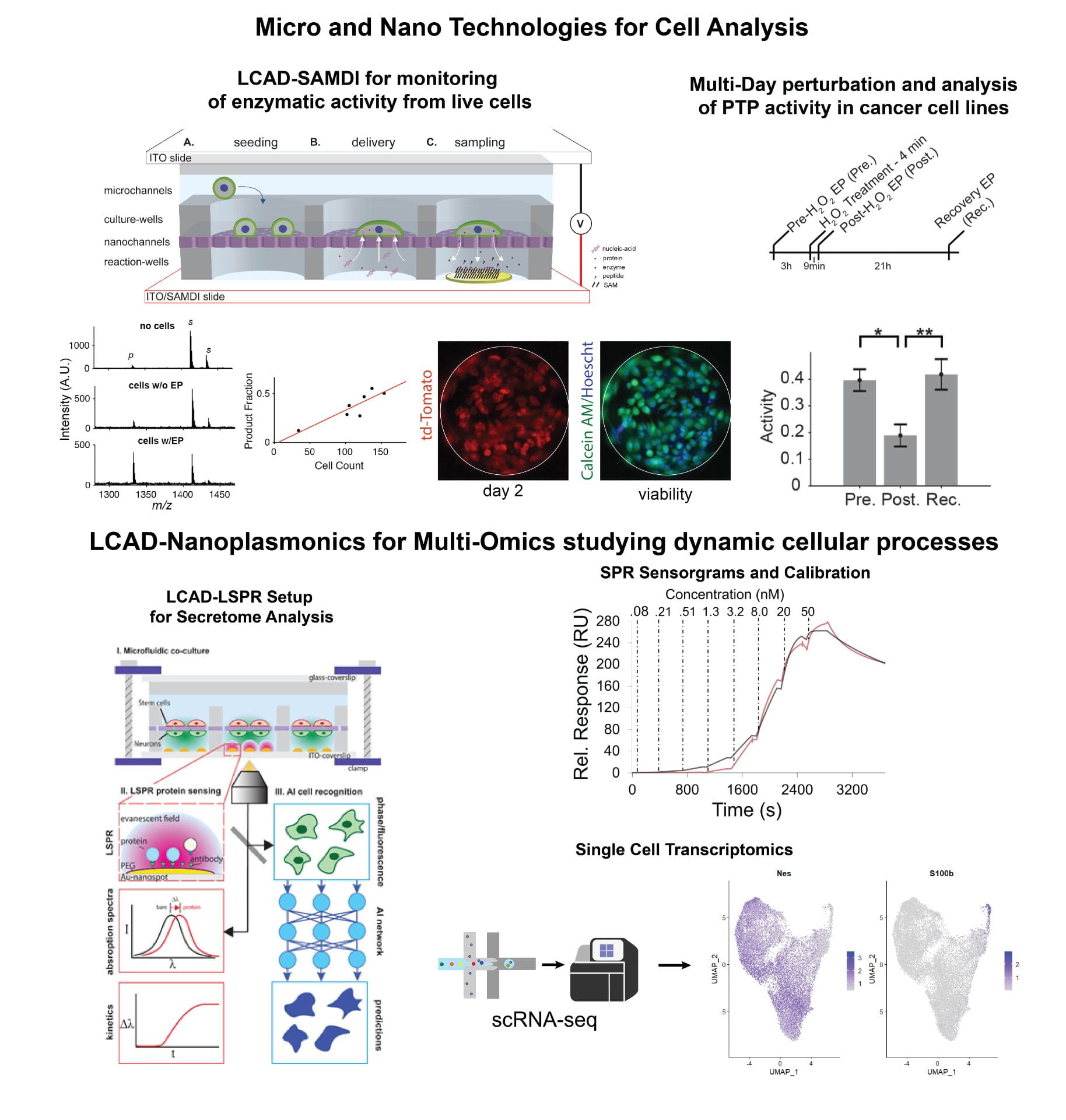
To address these challenges, we have established a minimally invasive method called localized electroporation that allows for controlled cellular perturbation with negligible impact on their structure and function. In this context, we have developed a localized electroporation-based microfluidic platform for high-throughput and multiplexed analysis of live cells, called the Live Cell Analysis Device (LCAD). The LCAD allows for intracellular delivery and temporal monitoring of cytosolic extracts or secretions that enables the study of dynamic cellular processes. Using the LCAD, we have demonstrated the extraction of intracellular/exogenous enzymes and analysis of their activity at multiple timepoints.
Current work is focused on multi-omics studies where the LCAD is combined with nano-plasmonic sensors for the analysis of cell secretome and is being used in conjunction with single cell transcriptomics to gain insights into dynamic processes such as cell differentiation.
BIOPHYSICS OF INTRACELLULAR DELIVERY
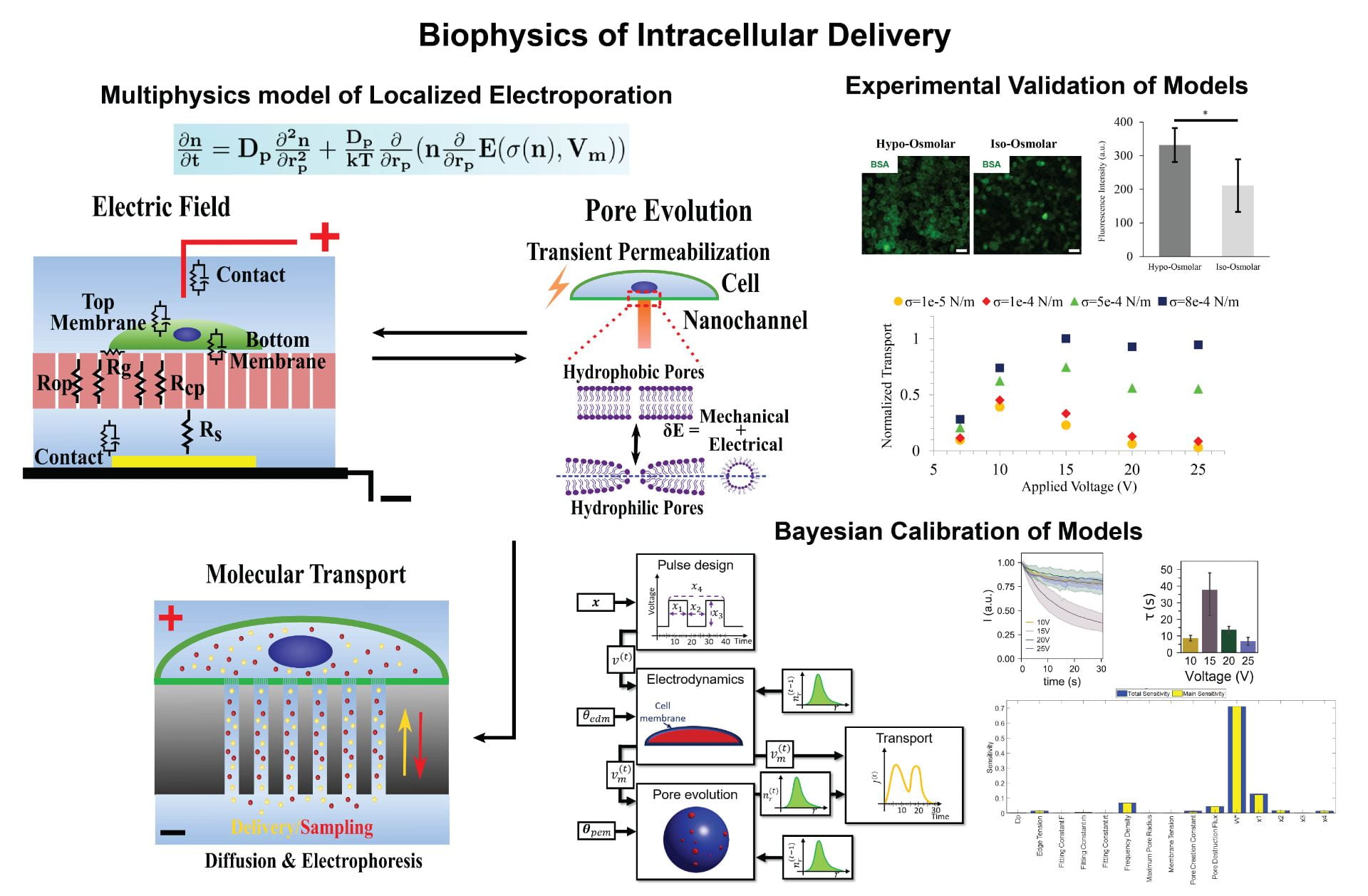
Localized electroporation has evolved as an effective technology for the non-toxic delivery of a multitude of foreign molecules into a wide variety of cell types. Although there have been numerous experimental studies on localized electroporation-based delivery, there is a lack of mechanistic understanding of the process, which makes its optimization, for different cell types and molecules, a labor-intensive task based on trial and error. To gain a mechanistic understanding of the process, we developed a multiphysics continuum scale model that predicts the transport of molecules in and out of the cell during localized electroporation. Using the model, we have identified that cell membrane tension plays a crucial role in enhancing both the amount and the uniformity of molecular transport, particularly for macromolecules. We have also identified that there exists an optimum for the electroporation pulse parameters that leads to efficient intracellular delivery. We have experimentally validated the model predictions using the localized electroporation device (LEPD).
Our current work is focused on investigating the process of localized electroporation mediated intracellular transport at greater detail using computational and experimental approaches across multiple scales. We are using a combination of continuum and molecular scale modeling, live cell tracking on the LEPD, super resolution imaging, and machine learning based parametric calibration. We aim at gaining a mechanistic understanding of the transport process and develop quantitative models of localized electroporation mediated molecular transport.
SELECT PUBLICATIONS
MICROFLUIDIC PLATFORMS FOR GENE EDITING
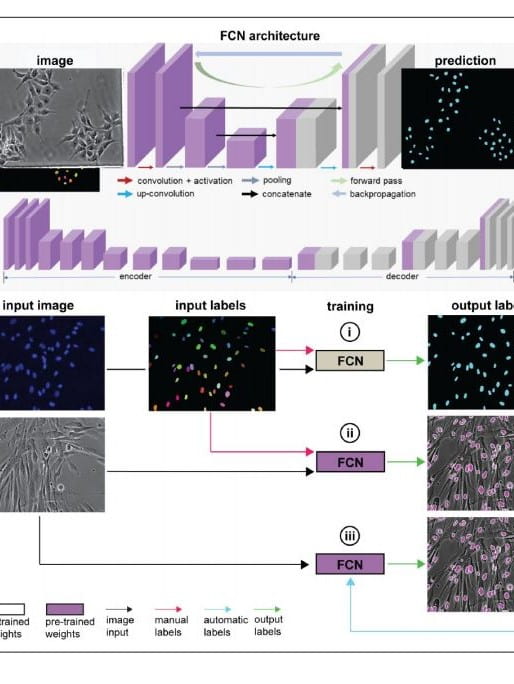
Deep learning and computer vision strategies for automated gene editing with a single-cell electroporation platform
C.A. Patino, P. Mukherjee, V. Lemaitre, N. Pathak, H.D. Espinosa†
SLAS Technology: Translating Life Sciences Innovation Vol. 26(1), 2021, pp. 1-11.
Single-cell delivery platforms like microinjection and nanoprobe electroporation enable unparalleled control over cell manipulation tasks but are generally limited in throughput. Here, we present an automated single-cell electroporation system capable of automatically detecting cells with artificial intelligence (AI) software and delivering exogenous cargoes of different sizes with uniform dosage. We implemented a fully convolutional network (FCN) architecture to precisely locate the nuclei and cytosol of six cell types with various shapes and sizes, using phase contrast microscopy…
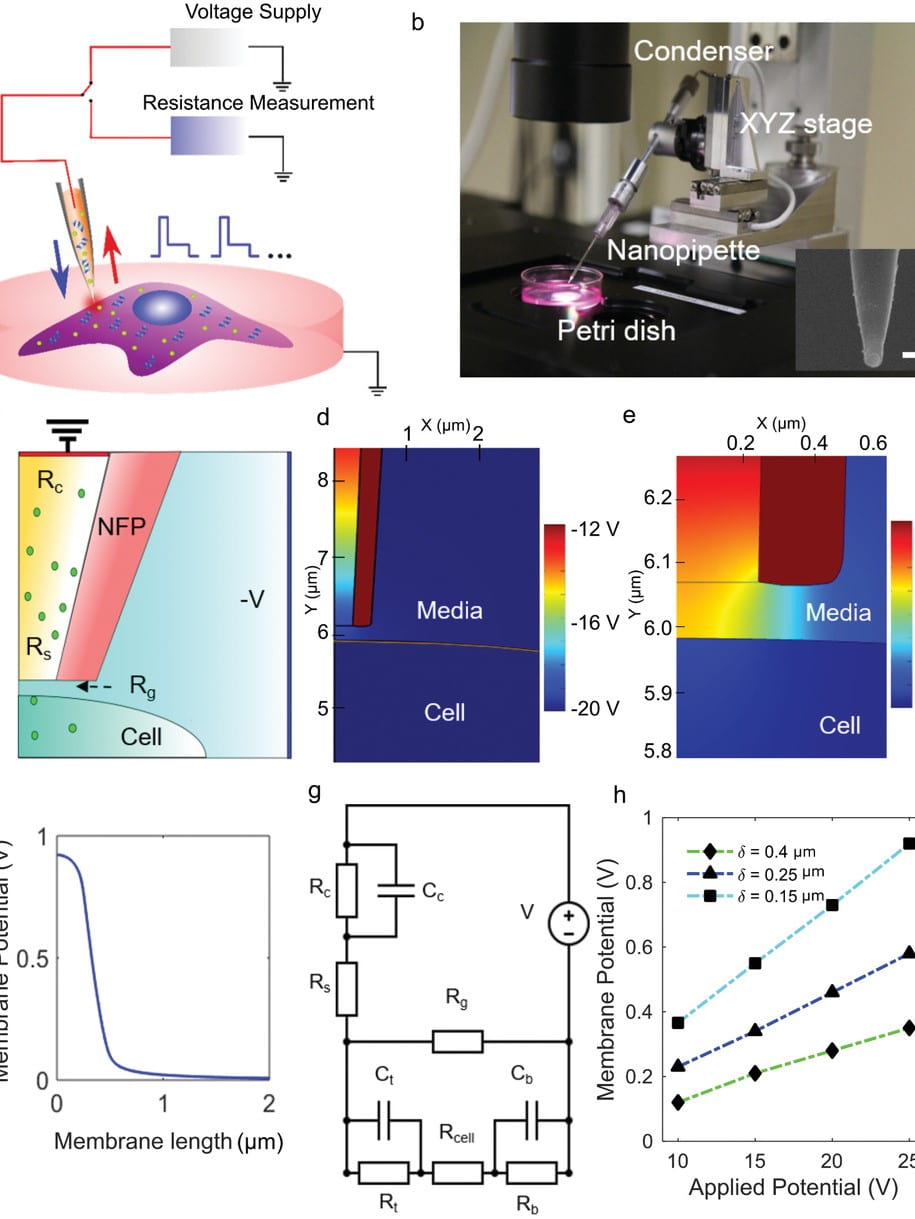
Nanofountain probe electroporation enables versatile single-cell intracellular delivery and investigation of postpulse electropore dynamics
S.S.P. Nathamgari*, N. Pathak*, V. Lemaitre*, P. Mukherjee, J.J. Muldoon, C.-Y. Peng, T. McGuire, J.N. Leonard, J.A. Kessler, H.D. Espinosa†
Introducing exogenous molecules into cells with high efficiency and dosage control is a crucial step in basic research as well as clinical applications. Here, the capability of the nanofountain probe electroporation (NFP‐E) system to deliver proteins and plasmids in a variety of continuous and primary cell types with appropriate dosage control is reported. It is shown that the NFP‐E can achieve fine control over the relative expression of two cotransfected plasmids. Finally, the dynamics of electropore closure after the pulsing ends with the NFP‐E is investigated…

Monoclonal cell line generation and CRISPR/Cas9 manipulation via single‐cell electroporation
R. Yang, V. Lemaître, C. Huang, A. Haddadi, R. McNaughton, H.D. Espinosa†
Small 14(12), 2018, pp. 1702495
Stably transfected cell lines are widely used in drug discovery and biological research to produce recombinant proteins. Generation of these cell lines requires the isolation of multiple clones, using time‐consuming dilution methods, to evaluate the expression levels of the gene of interest. A new and efficient method is described for the generation of monoclonal cell lines, without the need for dilution cloning. In this new method, arrays of patterned cell colonies and single cell transfection are employed to deliver a plasmid coding…

Single‐Cell Detection of mRNA Expression Using Nanofountain‐Probe Electroporated Molecular Beacons
J.P. Giraldo‐Vela, W. Kang, R.L. McNaughton, X. Zhang, B.M. Wile, A. Tsourkas, G. Bao, H.D. Espinosa†
Small 11(20), 2015, pp. 2345-2345
New techniques for single‐cell analysis enable new discoveries in gene expression and systems biology. Time‐dependent measurements on individual cells are necessary, yet the common single‐cell analysis techniques used today require lysing the cell, suspending the cell, or long incubation times for transfection, thereby interfering with the ability to track an individual cell over time. Here a method for detecting mRNA expression in live single cells using molecular beacons…
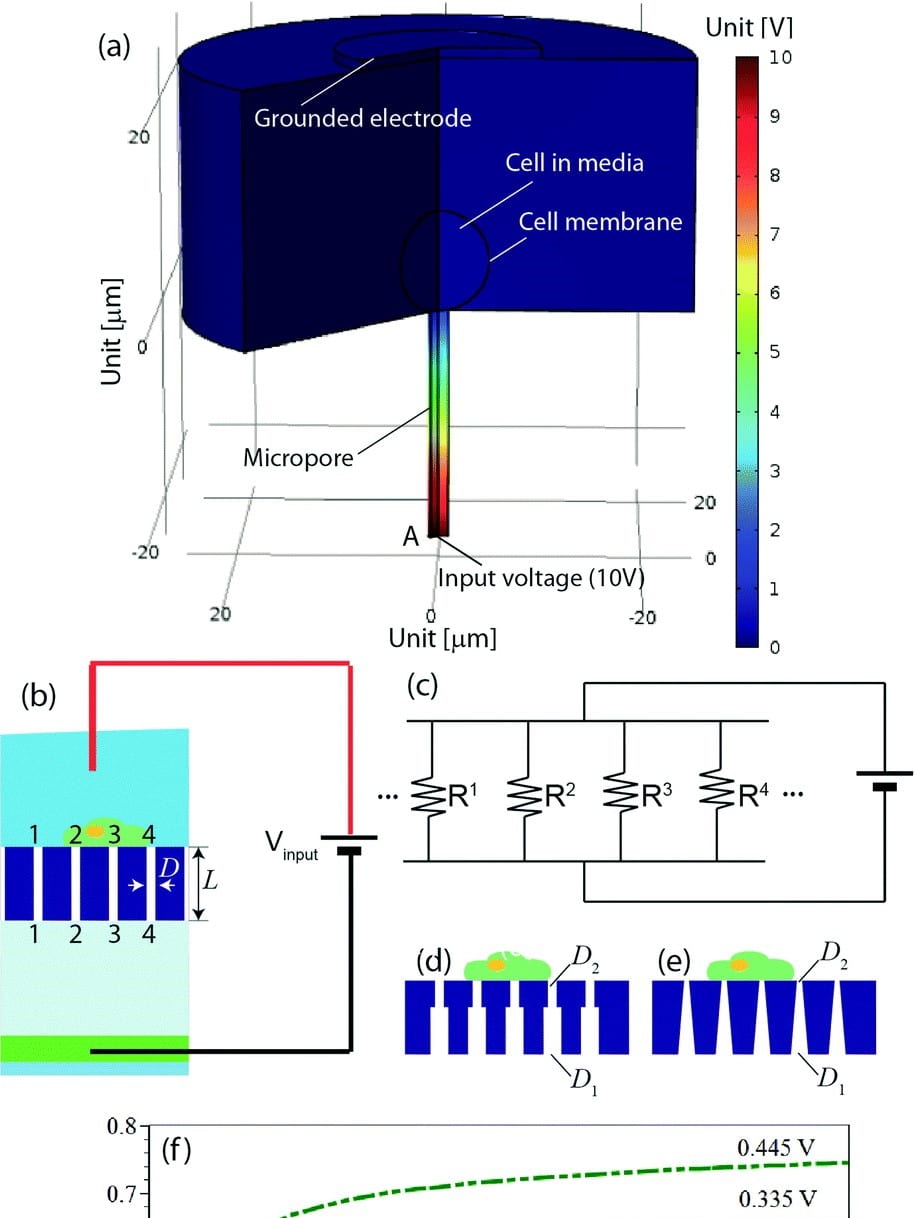
Microfluidic device for stem cell differentiation and localized electroporation of postmitotic neurons
W. Kang, J.P. Giraldo-Vela, S.S.P. Nathamgari, T. McGuire, R.L. McNaughton, J.A. Kessler, H.D. Espinosa†
Lab on a Chip 14(23), 2014, pp. 4486-4495
New techniques to deliver nucleic acids and other molecules for gene editing and gene expression profiling, which can be performed with minimal perturbation to cell growth or differentiation, are essential for advancing biological research. Studying cells in their natural state, with temporal control, is particularly important for primary cells that are derived by differentiation from stem cells and are adherent, e.g., neurons. Existing high-throughput transfection methods either require cells…
MICRO AND NANO TECHNOLOGIES FOR ANALYSIS
Temporal Sampling of Enzymes from Live Cells by Localized Electroporation and Quantification of Activity by SAMDI Mass Spectrometry
P. Mukherjee*, E.J. Berns*, C.A. Patino, E.H. Moully, L. Chang, S.S.P. Nathamgari, J.A. Kessler, M. Mrksich†, H.D. Espinosa†
Small 16(26), 2020, pp. 2000584
Measuring changes in enzymatic activity over time from small numbers of cells remains a significant technical challenge. In this work, a method for sampling the cytoplasm of cells is introduced to extract enzymes and measure their activity at multiple time points. A microfluidic device, termed the live cell analysis device (LCAD), is designed, where cells are cultured in microwell arrays fabricated on polymer membranes containing nanochannels. Localized electroporation of the cells opens…
BIOPHYSICS OF INTRACELLULAR DELIVERY
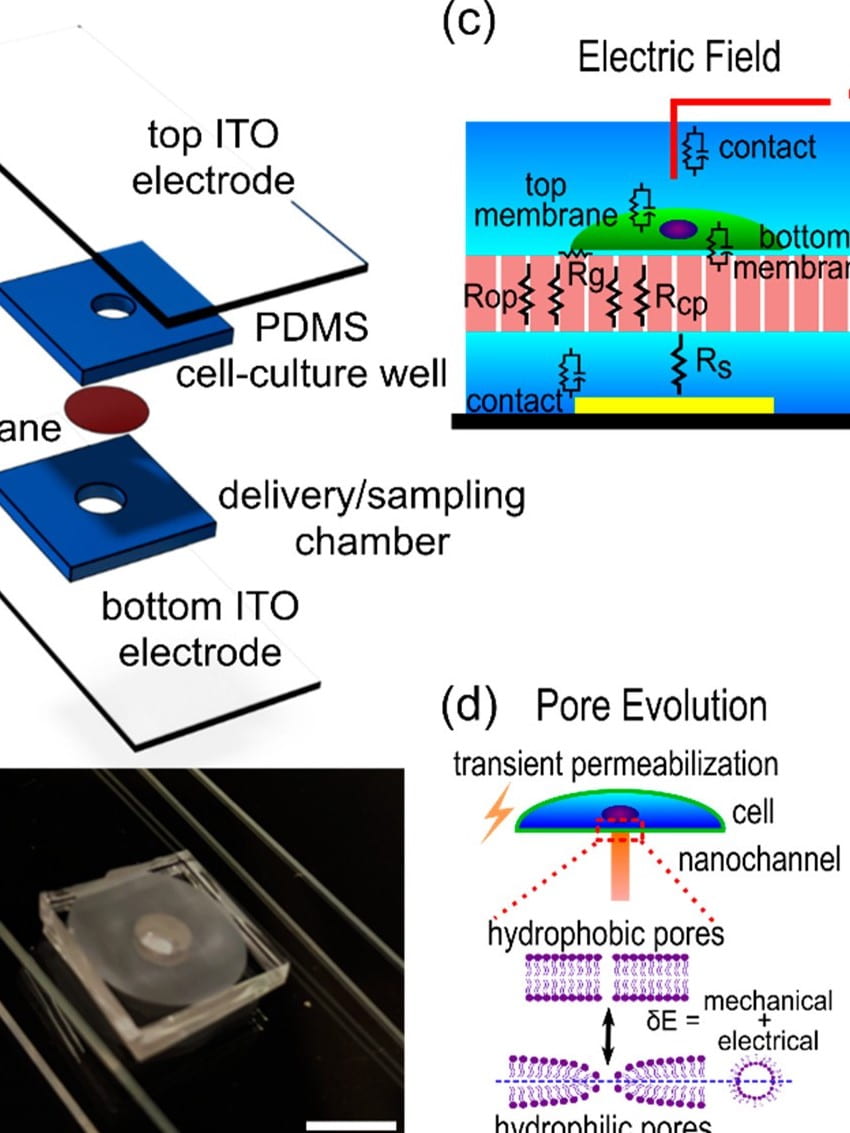
Combined numerical and experimental investigation of localized electroporation-based cell transfection and sampling
P. Mukherjee*, S.S.P. Nathamgari*, J.A. Kessler, H.D. Espinosa†
ACS Nano 12(12), 2018, pp. 12118–12128
Localized electroporation has evolved as an effective technology for the delivery of foreign molecules into cells while preserving their viability. Consequently, this technique has potential applications in sampling the contents of live cells and the temporal assessment of cellular states at the single-cell level. Although there have been numerous experimental reports on localized electroporation-based delivery, a lack of a mechanistic understanding of the process hinders its implementation in sampling. In this work, we develop a multiphysics model…